Results of the call Creation and Development of the Oncological Clinical Research Support Centres
The Medical Research Agency has provided more than PLN 47 million for the establishment of specialised Oncological Clinical Research Support Centres (OnkoCWBK), which will join the Polish Clinical Research Network. The OnkoCWBK competition focused on on oncological centres. The 7 centres recommended for funding follow the existing 16 centres selected by MRA in previous competitions and operating within the framework of the Polish Clinical Research Network.
The initiative to create Clinical Research Support Centres aims to make the market for clinical trials in Poland more attractive, thereby helping to improve patients' access to modern, innovative therapies and increasing the choice of sponsors of research centres in our country.
and the increase in the number of studies carried out. The newly established centres allow better coordination of clinical trials in Poland.
47 million for the creation of seven oncological Clinical Research Support Centres in the Polish Clinical Trials Network.
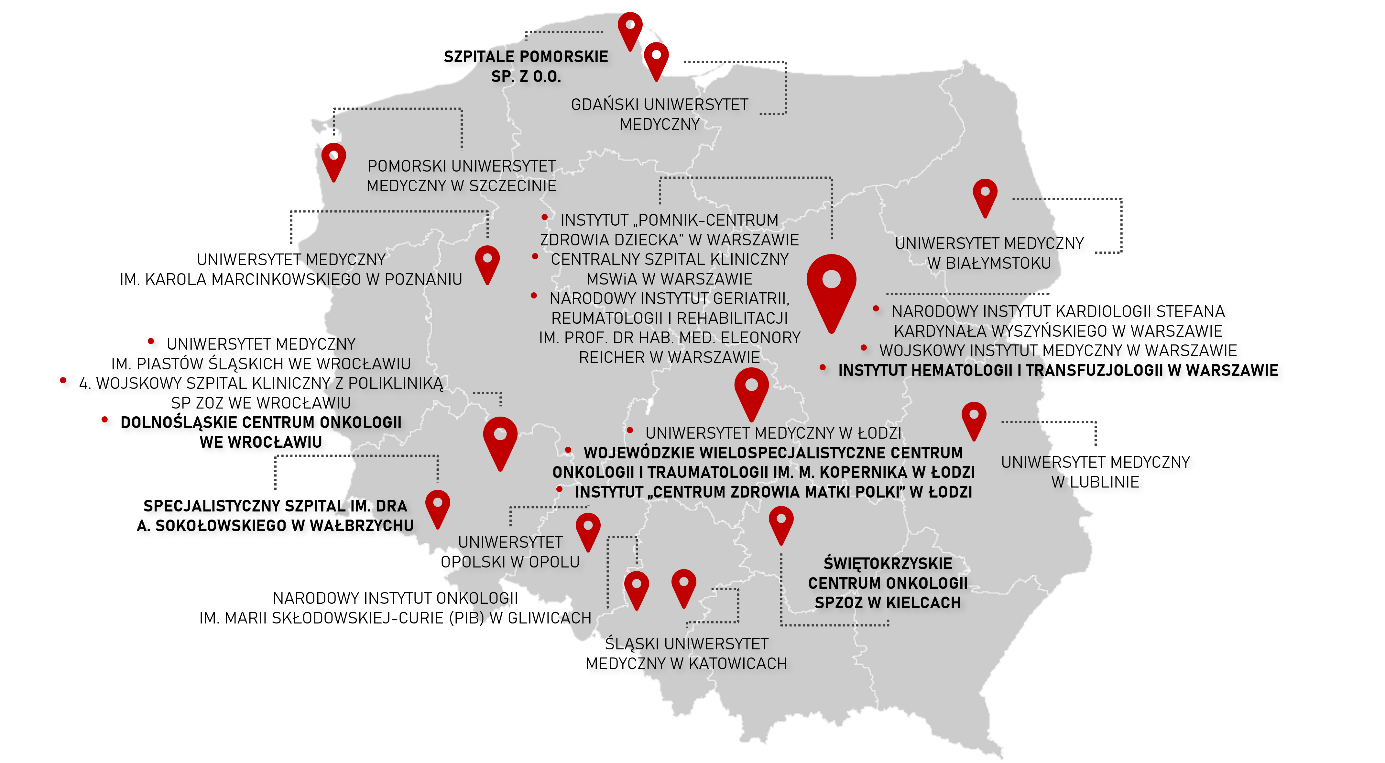
In the context of the call for the creation and development of Oncological Clinical Research Support Centres, the Medical Research Agency received 8 applications with a total amount of more than PLN 55 million. Among the beneficiaries selected in this competition were 7 organisations:
- Pomeranian Hospitals
- Multispecialist Centre for Oncology and Traumatology “M. Kopernikus” in Łódź
- Sokołowski Special Hospital in Wałbrzych
- Institute of Haematology and Transfusiology in Warsaw
- Lower Silesian Oncology Centre in Wroclaw
- Oncology Centre Independent public health facility in Kielce
- Institute “Polish Mother’s Health Centre” in Łódź.
These institutions have excellent scientific staff, an enormous volume of patients and often deal with very difficult cases.
National Oncology Network
The competition was planned taking into account the provisions of the National Oncology Strategy. This is particularly true for clinical trials in the early phases (I and II). One of the expected results of the implementation of this area is the creation of at least 4 Clinical Research Support Centres by 2024 and the increase of the number of Clinical Research Support Centres to 8 by 2029. The strategy foresees that by 2023: The Agency will support the development of the Clinical Research Support Centres at selected academic and oncological centres within the framework of the National Oncological Network.
Comprehensive support for Polish centres conducting clinical trials
Funding institutions will establish the Oncological Clinical Research Support Centres as a joint service centre providing comprehensive and systemic support for the conduct of commercial and commercial studies.
and not commercially. This support will cover, inter alia, the planning, management, accounting and coordination of studies and the upgrading of the qualifications of the staff involved. The infrastructure of OnkoCWBK will consist mainly of an area for outpatient visits, an area for patient relaxation, a conference room and rooms for the representatives of the sponsor.
Polish Clinical Research Network Uniformity, Standardization, Networking
The beneficiaris of the call will belong to a group of entities operating within the framework of the Polish Clinical Research Network established by the Medical Research Agancy.
The Polish Clinical Research Network aims to ensure a uniform standard for the conduct of studies, a high quality of patient care and an increase in the number of commercial and non-commercial clinical trials. The inclusion of the seven new OnkoCWBKs in the network will enable the creation of standardised centres that are not inferior to comparable structures in Western Europe. They will become the leading institutions in healthcare, conducting research at the highest level and taking care of the safety of study participants. Clinical studies in Poland are carried out in a friendly way for the sponsor, the hospital and the patient.
The first investments in facilities that have received an MRA grant in the competition are planned later this year. The starting signal for all projects to be financed is scheduled for 21. 03. 2022.