Regional Digital Medicine Centres (RDMCs)
WHAT ARE REGIONAL DIGITAL MEDICINE CENTRES (RDMCs)?
The Regional Digital Medicine Centres (RDMCs) are an initiative of the Medical Research Agency (MRA) to develop a digital infrastructure for biomedical research with a particular focus on clinical trials and the efficient management of large health and biomedical datasets. The centres are part of a nationwide Network of Digital Medicine Centres (DMCs), overseen by the MRA’s Digital Medicine Central.
Thanks to RDMCs, Poland is entering a new era of digital medicine, where innovative technologies and real-time data analysis support clinical research, diagnostics and patient care.
OBJECTIVES OF RDMCs
Development of infrastructure for clinical research:
- Creating and developing an advanced technological background to enable cutting-edge clinical research.
- Integration with existing Clinical Trial Support Centres (CTSCs) to maximize the potential for collaboration between units.
Effective health data management:
- Collecting and analysing a variety of health data: from imaging results to genetic data and information obtained from medical procedures and monitoring devices.
- Anonymisation and pseudonymisation of data in accordance with international security standards.
Real-time and retrospective data analysis:
- Enabling immediate responses to analytical results, improving the quality and safety of clinical trials.
- Conducting retrospective analyses of large datasets to discover new health trends and patterns.
Collaboration with academic, government and commercial sectors:
- Sharing anonymised data for research and commercial applications.
- Supporting scientific projects in the field of digital medicine and biotechnology.
Creation of a uniform medical data standard:
- Developing technical standards for data sharing and analysis.
- Ensuring consistency of procedures between different centres across the country.
SCOPE OF ACTIVITIES OF THE RDMCs
- Analysis of omics data: genetic, epigenetic, transcriptomic, proteomic, metabolomic.
- Integration of data from different sources: telemetry, monitoring devices, individual patient surveys and data on biological material stored in biobanks, clinical trials.
- Implementing artificial intelligence (AI): developing predictive models and diagnostic algorithms to support clinicians in making therapeutic decisions.
- Support for clinical trials: optimising study designs and ensuring the quality of results at every stage of implementation.
- Data security: applying strict safety procedures in accordance with EU and national regulations.
- Use of data from Hospital Information Systems (HIS): Analysing information including demographic data, disease classifications (ICD-10), procedures performed (ICD-9), administered medications (EAN code), and results of laboratory, imaging, and histopathological diagnostics.
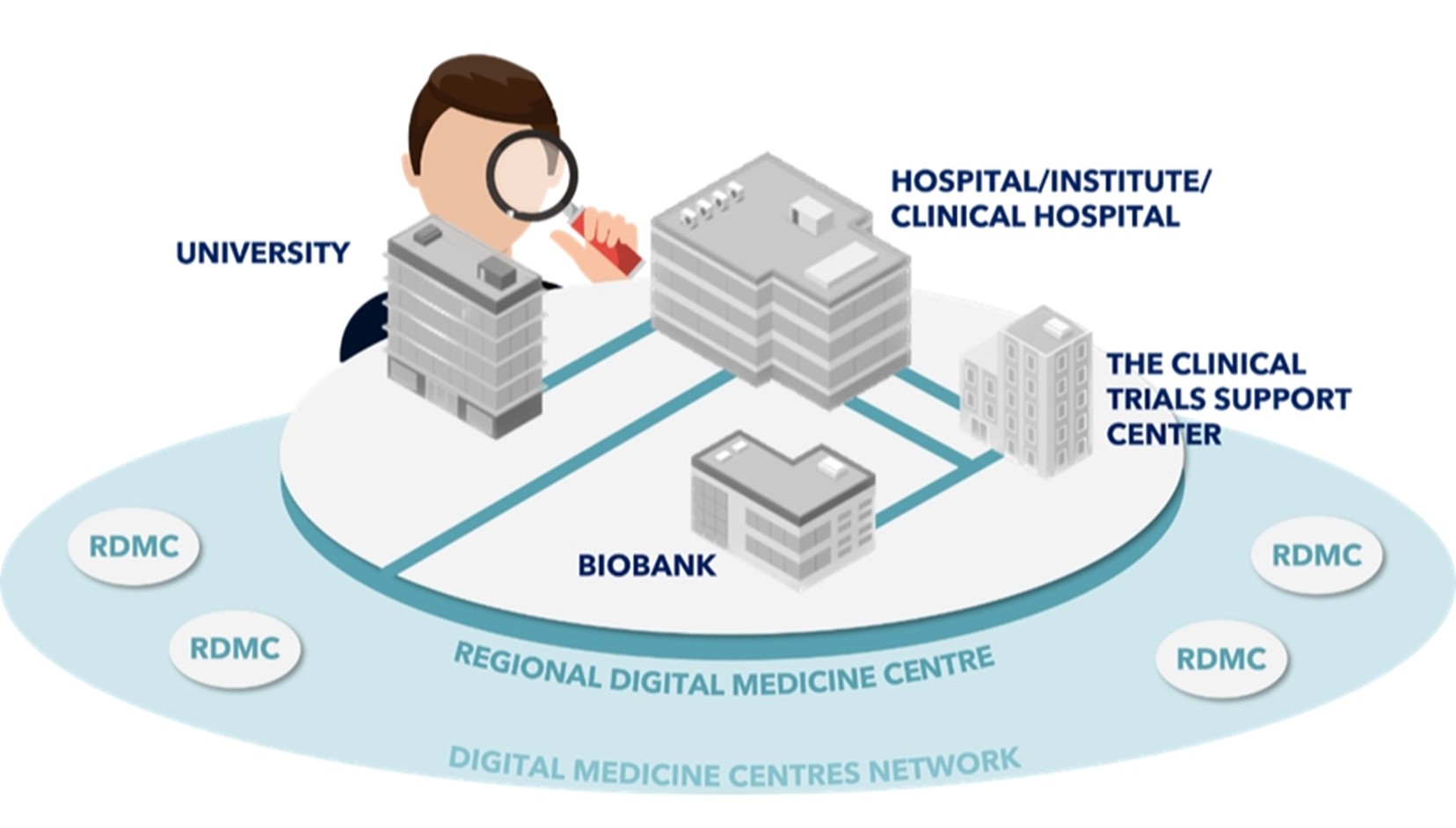
The figure shows the integrated collaborative system of the Regional Digital Medicine Centres (RCMCs). It connects key players - universities, teaching hospitals, clinical research support centres and biobanks - to form a network for effective health data management. By integrating diverse data sources, it is possible to forecast health trends, optimise medical care and support therapeutic and strategic decisions. The biobank plays a key role in collecting and making available the biological samples for scientific research.
The overall picture shown in the figure reflects a coherent digital medicine ecosystem, supporting the development of innovative therapies and improving the quality of healthcare.
MAP OF RDMCs IN POLAND
Regional Digital Medicine Centres (RDMCs) are strategically located in key academic and research hubs across the country. Each centre collaborates with local medical entities, other research institutions, and actively participates in the exchange of data and research results within regional, national, and international projects.
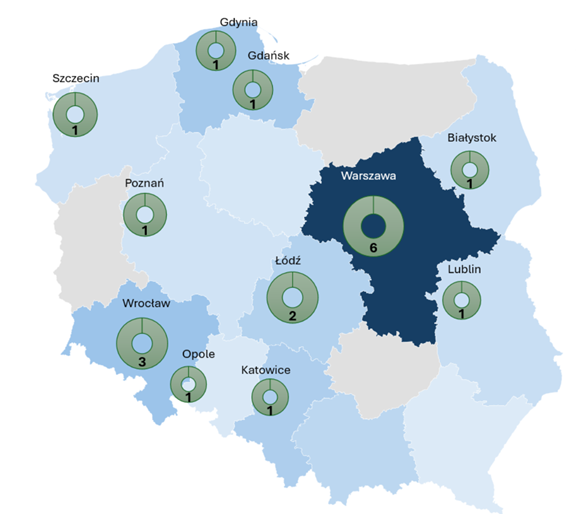
List of RDMCs :
GDAŃSK
- Medical University of Gdansk
GDYNIA
- Pomeranian Hospitals LLC
BIAŁYSTOK
- Medical University of Bialystok
WARSZAWA
- Cardinal Wyszynski National Institute of Cardiology
- National Medical Institute of the Ministry of the Interior and Administration
- The Children’s Memorial Health Institute
- Military Institute of Medicine – National Research Institute
- National Institute of Geriatrics, Rheumatology and Rehabilitation
- Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw
LUBLIN
- Medical University of Lublin
ŁÓDŹ
- Medical University of Lodz
- Voivodeship Multi-Specialist Centre for Oncology and Traumatology in Lodz
KATOWICE
- Medical University of Silesia in Katowice
OPOLE
- University of Opole
WROCŁAW
- Wroclaw Medical University
- 4th Military Clinical Hospital with Polyclinic in Wroclaw
- Lower Silesian Oncology Centre in Wroclaw
POZNAŃ
- Poznan University of Medical Sciences
SZCZECIN
- Pomeranian Medical University in Szczecin
BENEFITS OF COOPERATION WITH RDMCs
- Access to modern medical technology – advanced data analysis and artificial intelligence tools.
- Safe data management – the highest standards of data security and anonymisation.
- Implementation of innovative clinical trials – projects shaping the future of medicine.
- Cooperation with leaders in digital medicine – a network of experts, scientists and medical technology specialists.
- Participation in joint international projects – global scientific and technological cooperation.